GVK BIOSCIENCES P. LTD.
Featured Products
Highest quality standards are achieved through the implementations of latest technology, decades of experience and everlasting moral values , which have helped us to retain our customers as well as multiply them.
Welcome to GVK BIOSCIENCES P. LTD.
Chemical Development Services
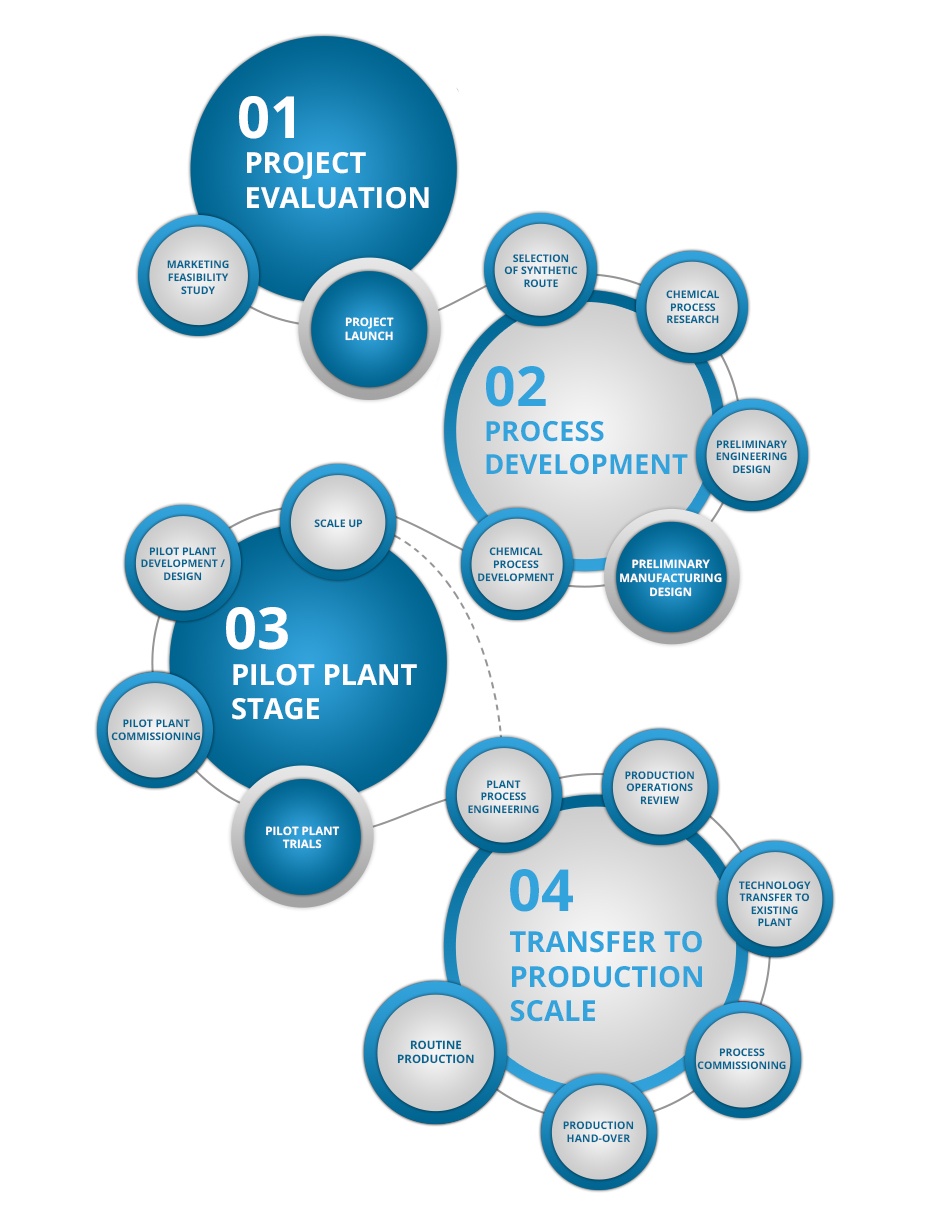
Description / Specification of Chemical Development Services
We are involved in offering a wide range of Chemical Development Services to our most valued clients. Our range of these are widely appreciated by our clients which are situated all round the nation. We offer our range of it at most affordable prices.
We introduce ourselves as an eminent trader and supplier of an extensive array of Chemical Development Services. Prior to dispatch, our quality controllers run numerous tests on the entire range on various parameters of quality and durability. The offered equipment is manufactured using superior quality components with the aid of sophisticated techniques at the vendors' end. Moreover, our precious clients can purchase it in different technical specifications at reasonable price from us within promised time frame.
Features:
Sturdy design
Highly durable
Application specific design
Formulations Services

Description / Specification of Formulations Services
We are involved in offering a wide range of Formulations Services to our most valued clients. Our range of these are widely appreciated by our clients which are situated all round the nation. We offer our range of it at most affordable prices.
We introduce ourselves as an eminent trader and supplier of an extensive array of Formulations Services. Prior to dispatch, our quality controllers run numerous tests on the entire range on various parameters of quality and durability. The offered equipment is manufactured using superior quality components with the aid of sophisticated techniques at the vendors' end. Moreover, our precious clients can purchase it in different technical specifications at reasonable price from us within promised time frame.
Features:
Sturdy design
Highly durable
Application specific design
Analytical Services

We offer a broad range of analytical services for complex research needs. We perform method development, validate and transfer Good Manufacturing Practice (cGMP) compliant methods for a broad spectrum of pharma compounds. We also perform stability analysis of drug products and active ingredient (APIs), packaging as per ICH, quality control testing and release as per cGMP requirements.
Our team is capable of providing documentation support – CMC, IND-CTA, MAA and DMF in line with the regulatory requirements across the globe.
Equipped with one of the best infrastructures in India for Analytical services, GVK BIO is well positioned to meet the needs of complex analytical challenges.
GET IN TOUCH WITH OUR SCIENTIFIC TEAM
Our capabilities:
A wide range of chromatographic separation techniques (HPLC, UPLC, GC, SFC and IC) and detection techniques (UV, FL, MS, ELSD, RI, FID, TCD, CAD, etc.) to meet the requirement of different types of compounds including the ones that are non-chromophoric in nature.
Stability-indicating assay and/or related substances methods for drug substances & products (stress stability testing as per ICH).
Dissolution (IR, ER and MR)
Residual solvents by HSGC
Enantiomeric separation by normal phase HPLC & GC
Clinical comparator assay and dissolution
Method development, qualification, validation and transfer
Release and in-process testing
Stability protocol development and program management
Stability storage, testing and data trending
Compendial testing (USP, EP, BP, JP, etc.)
Microbiological testing
Analytical support for cleaning validation & process validation
Isolation of impurities and purification of compounds using Preparative LC & SFC
Reference standard characterisation & qualification
Materials characterization
Thermal analyses
Particle-size distribution
Spectroscopy (NMR, Mass, FTIR, GC-MS, UV, etc.)
GVK BIO supports extensive structural chemistry services for small and large molecules that include:
Impurity and degradation product identification and structural elucidation
Characterization of API, product, reference standard and other pharmaceutical ingredients
Molecular weight determination for large biomolecules
Support for regulatory documentation for IND, MAA, DMF, etc
Analytical reference standards qualification
[analytical-rd]
Method Development
Robust analytical method is one of the keys to quality product development and faster regulatory approval. At GVK BIO, we develop product specific analytical methods, which are best suitable for intended use of application with an approach of ‘right first time’. We help our partners globally to develop various analytical methods at efficient cost and meeting global quality and regulatory standards.
The Analytical Services team has expertise in developing analytical methods for:
Reverse engineering
Identification
Assay
Related impurities
Chiral purity
Preservative anti-oxidant content
Residual solvents
Particle size distribution
In-vitro dissolution
Physicochemical and wet chemical methods
Various advanced and conventional analytical techniques & instruments are adopted for developing analytical methods like:
Chromatography (HPLC, UFLC, GC and TLC)
Spectrophotometry (UV-visible)
Laser Diffraction Technology (Particle Size Analyser)
Potentiometry (Auto & KF titrator)
USP-1 and USP-2 Dissolution tester (Automated & Manual)
Titrimetry
Our analytical services team develops all methods based on a thorough scientific literature search and review, which leads to appropriate selection of analytical technique and development design for the targeted product/analyte. Performance of Instruments is ensured throughout the experimentation, which is handled by highly trained and skilled analysts. Robustness testing and key preliminary validation performance parameters are integral to our development design method.
We excel in development of In-Vitro dissolution methods, which plays a critical role in drug development process especially for Solid oral dosage forms where absorption of drug is necessary. We take great care while establishing a discriminating dissolution method with emphasis on following key elements:
Dissolution media selection based on pharmacokinetic parameters
Relevance to In-vivo performance
Sink conditions
BCS Classification of API
Selection of relevant analytical technique
Method Validation
Method validations are carried out as per latest regulatory guidelines for assay, related impurities, chiral purity, preservative & anti-oxidant content, residual solvents, particle size distribution, in-vitro dissolution, physicochemical and wet chemistry methods.
At GVK BIO, Analytical method validations are executed in alignment with stringent SOP, which is based on ICH Q2 guidelines and in adherence to cGMP & GLP requirements.
Validation experimentation includes:
Specificity
Forced degradation studies
Precision
Accuracy
Linearity
Quantitation limit/Detection limit
Stability of analyte in solution
Robustness studies
System suitability
Good documentation practices are followed throughout the process and an exhaustive and scientifically documented validation report is provided to the customers.
Forced Degradation Studies
Forced Degradations at the method development stage provides us useful information about drug product characteristics and also establishing stability indicating behaviour of analytical method.
At GVK BIO, forced degradation studies are an integral part of our method development and validation in order to establish stability indicating behaviour of methods and product characteristics. Forced degradation studies include exposure of analyte to harsh hydrolytic, oxidative, photolytic, thermal and humidity conditions.
Method Transfer
We ensure successful method transfer to our client manufacturing sites with:
Validated robust analytical method
Scientific rationalised scope with predefined limits
Active involvement from qualified analysts
All the analytical methods are transferred through protocol bound study in accordance with latest regulatory
guidelines such as USP (1224).
Formulation Development

Description / Specification of Formulation Development
We are involved in offering a wide range of Formulation Development to our most valued clients. Our range of these are widely appreciated by our clients which are situated all round the nation. We offer our range of it at most affordable prices.
We introduce ourselves as an eminent trader and supplier of an extensive array of Formulation Development. Prior to dispatch, our quality controllers run numerous tests on the entire range on various parameters of quality and durability. The offered equipment is manufactured using superior quality components with the aid of sophisticated techniques at the vendors' end. Moreover, our precious clients can purchase it in different technical specifications at reasonable price from us within promised time frame.
Features:
Sturdy design
Highly durable
Application specific design
A.P.I. Contract Manufacturing

GVK BIO offers long term Contract Manufacturing services right from strategic partnerships in development, validations, DMF filing and commercial manufacturing of Active Pharmaceutical Ingredients (APIs)/advanced intermediates to APIs. We have flexible business models to support various client needs:
Process optimisation, technology transfer, validations and DMF filing followed by manufacturing upon commercialization
Technology absorption, validations, DMF filing support and Commercial manufacturing
Technology absorption and commercial manufacturing
We have a diverse scientific team with expertise to meet API and advanced intermediate needs centred on the rapid synthesis of supplies. Our project management and technical teams ensure a seamless technology transfer of processes, as projects advance from feasibility to DMF filing through complete process optimisation, process validations and stability program. We can optimise and develop robust and cost efficient processes for large scale manufacturing using environment friendly routes under GMP conditions with globally acceptable regulatory guidelines.
The combination of development experience, manufacturing infrastructure and regulatory expertise makes GVK BIO a preferred strategic partner for reliable, cost effective, long term contract manufacturing partners for APIs/advanced intermediates.
GVK BIO’s manufacturing facility has approvals from worldwide regulatory authorities like USFDA, EDQM, PMDA, MFDS and WHO GMP. These accreditations facilitate faster and easier approvals of DMFs/dossiers for our business partners.
Our dedicated late stage development teams have extensive experience in the following:
Commercial synthesis route scouting
Commercial process development and optimisation through DoE and QbD
Stepwise unit operation studies and defining critical process parameters
Impurity profiling and define control measures
Documentation for all kinds of regulatory filings
Our commercial manufacturing facility is co-located with our pilot plant, providing end-to-end API service from feasibility phase in R&D to commercial launch. This integration eliminates the need for tech transfers when a project advances through each development phase, allowing us to help reduce delivery time while providing cost-effective services.
Our facilities can scale up all types of reactions, including asymmetric hydrogenations, air and water sensitive metal catalytic reactions, high temperature, high pressure, cryogenic and oxidations reactions.
The manufacturing facility has wide range of equipment:
Reaction vessels starting from capacity 20 L to 6000 L
Material of construction: stainless steel, glass lined, glass, halar coated, hastelloy-C, etc.
Utilities ranging from -70°C to +200°C
Supporting equipment to handle all kinds of unit operations
GVK BIO’s manufacturing team has expertise to handle a wide range of operating conditions with flexible scales. Over the years we have exhibited exemplary process and technology capabilities for NCEs and commercial production of advanced intermediates/APIs. Our process development, technology transfer and commercial execution teams collaborate seamlessly to provide best-in-class concept to commercialisation timelines.
Salient features:
Driven by the need to design an economic and robust process with defined in-process controls to yield optimum and desired quality
Provide engineering support from initial stages of product development by identifying process specific facility requirements to deliver a validated process at designated scale
Observe and assimilate process operations and all critical parameters and systematically execute under guidelines charted by Quality Management Systems
Manufacturing Infrastructure:
GMP compliant six manufacturing blocks with varied capacities ranging from 20 L – 6,000 L
Total reaction volume capacity: 170,000 L
MOC: All glass, glass lined, SS & Hastelloy reactors
Hydrogenation capabilities: 50 L to 2,000 L, upto 25 bar and large scale column chromatography
Class 100,000 cleanroom, kilo labs and powder processing area
Stability chambers for conducting stability studies at all conditions
Commercial A.P.I.

GVK BIO provides the following high-quality Commercial API and Intermediates:
Commercial APIs
S.No.
Products
Pharmacopoeia
Therapeutic Category
Documents Availability/Regulatory status
1 Dobutamine hydrochloride Ph. Eur./USP/BP/IP Cardiovascular CEP, Chinese DMF under filing
2 Trimetazidine dihydrochloride Ph. Eur./USP/BP/IP Anti-anginal CEP
3 Ezetimibe In-house/USP/JP/IP Anti-Hyperlipidemic DMF, Russian Registration
4 Lamotrigine Ph. Eur./USP/IP Anti-Convulsant CEP
5 Moxifloxacin hydrochloride monohydrate Ph. Eur. /USP/BP/IP Antibiotic CEP, WHO- APIMF, Ukraine Registration
6 Tramadol hydrochloride Ph. Eur. /BP/IP/USP Analgesic USDMF, CEP, KDMF & Japan DMF
7 Carvedilol Ph. Eur. /JP Anti-hypertensive JDMF
8 Pentosan polysulfate sodium In-house Interstitial Cystitis USDMF Under Compilation
9 Etoricoxib In-house/ IP Anti-Inflammatory, Analgesic DMF
10 Nebivolol hydrochloride In-house/ IP Anti-hypertensive DMF
11 Betahistine dihydrochloride Ph.Eur./USP/IP Anti-vertigo DMF
12 Linezolid (Form-II) In-house Antibiotic DMF
13 Tadalafil Ph. Eur. /USP Erectile Dysfunction DMF Under Compilation
14 Teneligliptin hemipenta hydrobromide hydrate In-house Anti-diabetic DMF Under Compilation
15 Vildagliptin In-house Anti-diabetic Under Development
Disclaimer: Products covered by valid patents in any country are not offered or supplied to those countries. The customer should verify the patent position in the concerned country.
API Intermediates
S.No.
NAME OF THE INTERMEDIATE
CAS NO.
Carvedilol
1 4- (2,3 –EpoxyPropoxy)-Carbazole (EPC-3/CVD4) 51997-51-4
Ezetimibe
4 3-[5-(4-fluorophenyl)-1, 5-dioxopentyl]-4-phenyl-(4S)-2-Oxazolidinone (FHO2) 189028-93-1
5 4-((4-fluoro phenylimino)methyl)phenol (FIP) 3382-63-6
6 {2-[3-(fluorophenyl)-3-(trimethyl siyloxy)-propyl]-3-(4-fluoro phenyl amino)-3-(4-trimethyl silyoxy phenyl)-1-oxo-propyl}-4-(S)phenyl oxazolidin-2-one (EZB1) 272778-12-8
Lamotrigine
8 2,3-Dichloro benzoyl Nitrile (LMG1) 77668-42-9
9 Hydrazine carboxidazole 2 – [cyano (2,3 – dichlorophenyl)]methane (LMG2) 84689-20-3
Lansoprazole
10 2-(3-methyl-4-(2,2,2-trifluoroethoxy)-2-pyridinyl) methylthio -1h-benzimidazole (Lansoprazole sulfide) 103577-40-8
Nebivolol
12 (αR,α’S,2S,2’S)-rel-α,α’-[[(phenylmethyl)imino]bis(methylene)]bis[6-fluoro-3,4-dihydro-2H-1-benzopyran-2-methanol] 876666-07-8
Our Vision
* To embrace new technologies and methods. * To give unsurpassed products and services to the clients. * To constantly look for improvement and changes.









